We prepare mg to multi-g amounts of fully characterized peptides for research. Most syntheses are achieved using solid-phase resin and Fmoc chemistry whilst solution-phase approach is utilized where appropriate for more demanding synthesis or special structural features. We further purify your peptides using reversed-phase HPLC to different levels of purities. We will assist you and recommend the optimum purity level required for your particular application. Peptides are sold for in vitro research use only and they are not intended for medical or diagnostics applications.
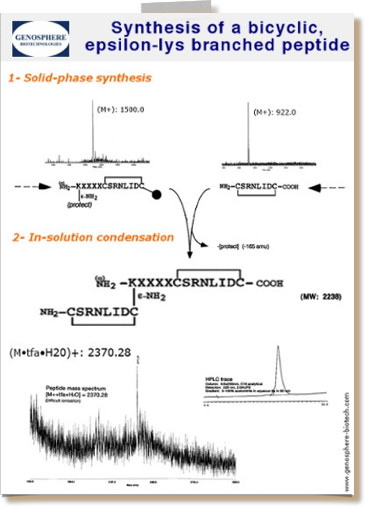
Synthesis plan for the synthesis of a bicyclic, branched 21 AS peptide, which includes the combination of solid-phase chemistry and liquid-phase chemistry.
1• Standard peptide synthesis scales and features
The table describes our classical, most frequently requested syntheses. Please contact us with your sequence(s), amount and purity level and we will always respond with a very competitive offer.
Custom peptide synthesis:
• Fast turnaround
• 97%+ success rates
• Guaranteed quality
| Pricing groups(I-VI) depending on synthesis scales (mg) | (I) (II) (III) (IV) (V) (VI) |
2 mg (minimum amount) 5 mg to 30 mg in increments of 5 mg 30 mg to 100 mg in increments of 10 mg 0.1 g to 1 g in increments of 50 mg 1 g to 10 g in increments of 500 mg >10 g |
| Purity levels: | >70%, >80%, >90%, >95% and >99% |
| Analytical Data: | HPLC trace (220 nm), mass spectrum (EI, MALDI-TOF) & dissolution conditions |
| Peptide Length: | Standard:5-30 aa ; Routine: 31-60 aa ; Maximum length about 100 aa |
| Structure: | Unblocked termini (i.e. NH2- and -COOH ends) |
| Counter ions: | Standard: Trifluoroacetate (tfa); Option: acetate or chloride |
| Form: | Lyophilized powder |
2• Custom peptide reverse phase HPLC purification
HPLC purification options include guaranteed purities >70%, >80%, >90%, >95% and a unique ultra-pure >99% grade for demanding experiments.
Purification is performed by preparative or semi-preparative reversed-phase HPLC. We typically use C8 or C18 columns with linear gradients of acetonitrile in 0.1% aqueous tfa with UV monitoring at 220 nm.
HPLC elution may be monitored with UV detector set at longer wavelength for peptides containing aromatic amino acids (Figure 2).
The crude peptide synthesis contains by-products resulting from failed coupling cycles (i.e. truncated chains), and further oxidation, modification, or hydrolysis of side chains arising during cleavage and deprotection. The extent of impurities varies with peptide length and primary sequence.
Depending upon your specific application we would recommend purification levels along the following guidelines.
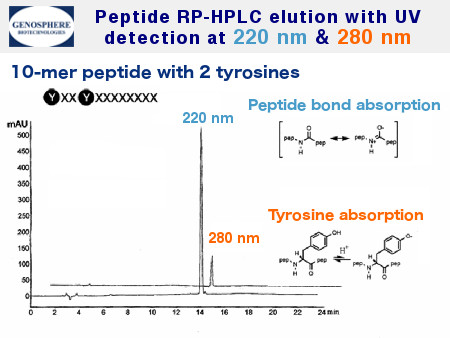
Reverse phase HPLC (RP-HPLC) analysis of a 10 AS peptide containing two aromatic amino acids (Tyr). The UV absorbance is recorded simultaneously at two different wavelengths: 220 nm and 280 nm. The simultaneous increase in both UV absorbance values confirms the purity of the peptide. C8 column 4.6x250mm, 5μm. Gradient 15-50% acetonitrile in 0.1% aqueous TFA in 25 min, flow rate 1ml/min.
Experimental applications and recommended purity levels
Purity >70%
Wide range peptide screen ; Polyclonal antibodies production; preliminary library screenings; higher levels should be considered for peptides > 20aa.
Purity >80%
Biochemical screening assays ; Qualitative immunoassays (ELISA, competition, depletion…) ; Polyclonal antibodies production with long peptides;
Purity >90%
Initial bioassays of candidate peptide ; Polyclonal mono-specific antibodies; affinity column making; structure-activity studies; qualitative biochemical assays (enzyme activity, specificity…).
Purity >95%
Quantitative immunoassays (ELISA, competition, depletion…); quantitative biochemical assays (enzyme activity, kinetics…); generally recommended with modified peptides; preliminary structural analysis (NMR,…).
Purity >99%, ultra-pure
Sensitive bioassays; cell culture assays; quantitative bioassays; structural studies (Crystallography, NMR,…).
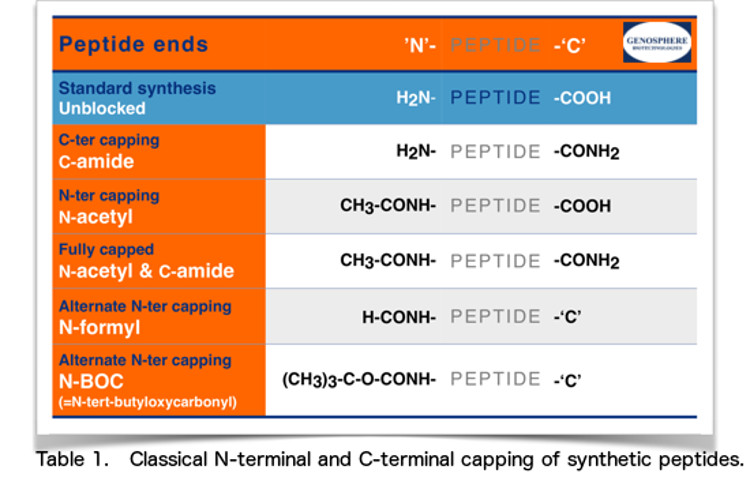
3• Peptide ends
The N-terminus is the end of a peptide that has a free amino group (-NH2), while the C-terminus is the end that has a free carboxyl group (-COOH). When designing a peptide, one must consider what ends would be best fitted for the particular application. Depending on the requirements, if any, for over whole peptide charge or stability towards exopeptidases activities, one might consider N- and/or C-capping.
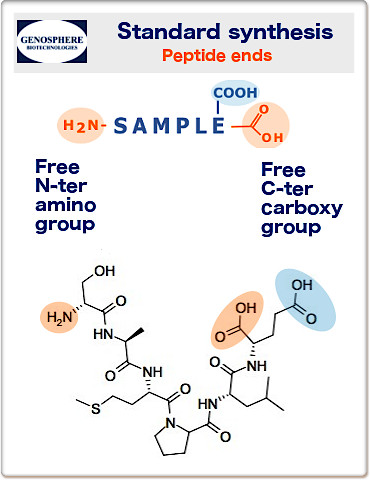
• Standard synthesis peptide ends:
N-amine and C-carboxyl
Our peptides are normally synthesized with unblocked termini:
N-terminal amine and C-terminal carboxyl groups.
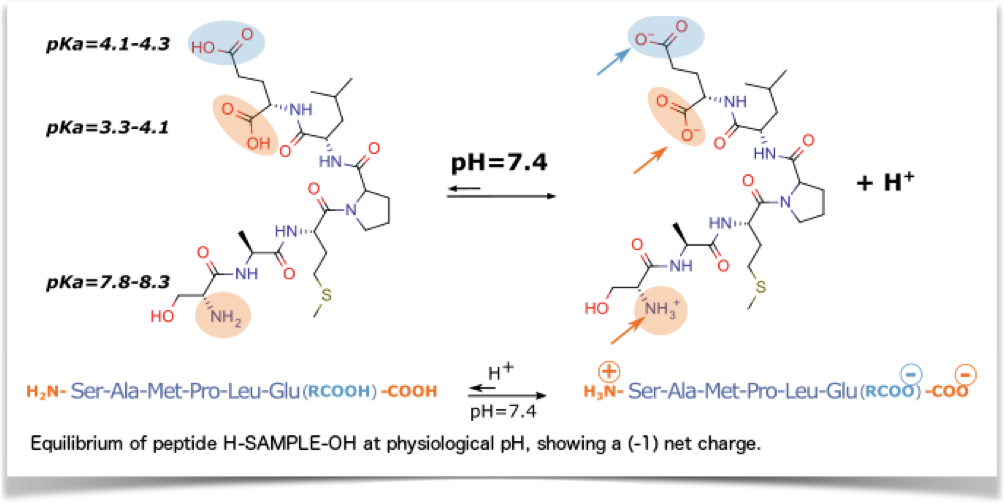
The pKa values of the terminal alpha-amino group and the carboxylic acid group indicates that the peptide ends are generally electrically charged at physiological pH.
• Capped peptide ends
In some instances, it may be preferable to neutralise these electric charges using capping groups. To that effect, peptide ends are often modified by:
N-terminal acetylation and/or C-terminal amidation.
N-acetyl and C-amide closely mimic internal sequences as they have uncharged ends. It should be noted that as expected, this charge neutralization also often results in decreased solubility.
In other cases, it may be desirable to modify the N- or C-terminus in a way that prevents or reduces enzymatic hydrolysis notably when the peptides are intended for use in biological systems where enzymatic activity is present e.g. body and cell fluids, bacteria, etc.
Peptidases can be grouped according to the pattern of proteolysis as either endo- or exo-peptidases. Exopeptidases catalyse the removal of amino acids (or short peptides) from the end of a polypeptide chain through the hydrolysis of peptide bonds.
These enzymes are widely distributed in nature and can be further divided in subgroups. Specifically, aminopeptidases cleave amino acids from the N-terminus of a peptide, while carboxypeptidases cleave amino acids from the C-terminus.
There again, N-acetylation and C-amidation are commonly utilised to protect the ends of the peptide from exopeptidase hydrolysis.
Other N-terminal modifications including N-pyroglutamylation, lipidation, cyclisation, and PEGylation have been found to increase bio-stability of peptides.

4• Counterions
Peptides are charged molecules. Standard reversed-phase HPLC purification allows for the isolation of pure peptides that are further freeze-dried yielding peptides preparations that comprise trifluoroacetate (= tfa = CF3COO-) as counterion.
TFA exchange services
Some applications require the removal of tfa. We offer ion exchange option to acetate (CH3COO-) or chloride (Cl-) salts.
5• Quality control: guaranteed peptides
We recognise that quality and reliability are central to our customers. As a result no peptide leaves our laboratories unless it has fully complied with our stringent QC standards every step of the process.
Standard synthesis report includes analytical HPLC trace verifying purity, a mass spectrum ensuring integrity of the desired full-length peptide and dissolution conditions.
Additional analyses may be performed as required:
• Elemental analysis for net peptide content
• tfa content
• Amino-acid analysis
• Water content
• NMR, CD, and other advanced techniques
Your delivered peptides are 100 % guaranteed.