Our company has gained extensive experience in manufacturing research scale difficult and sophisticated peptides. Our synthetic peptides have proven to be generally very stable and to exhibit high biological activity in a wide range of research areas.
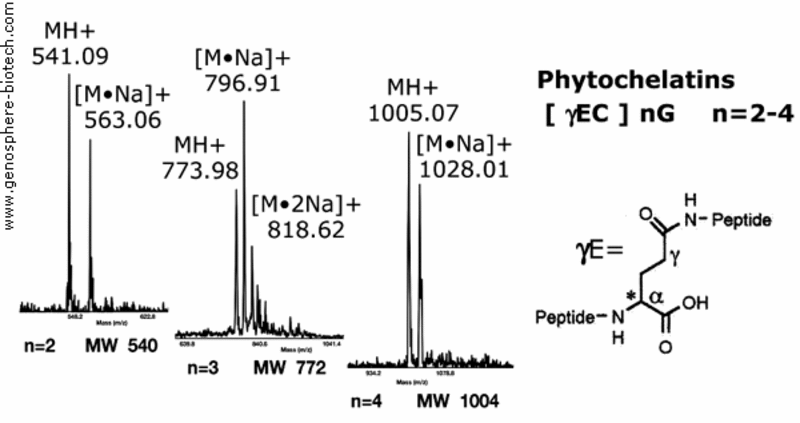
Mass spectra of synthetic metal binding phytochelatins plant peptides.
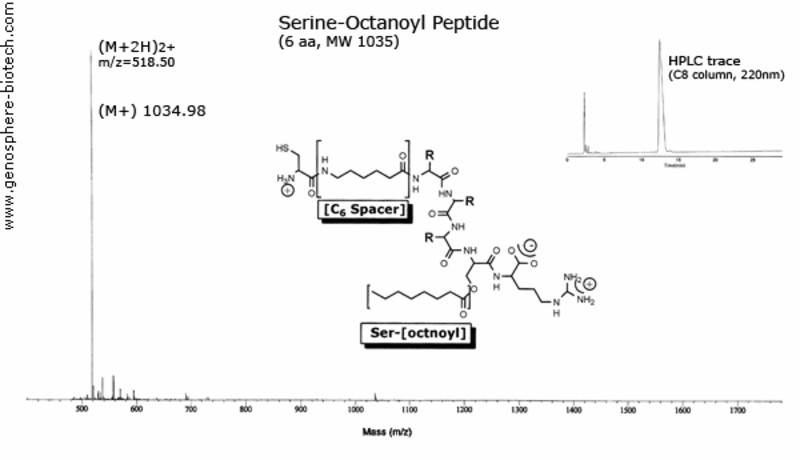
Mass spectrum and HPLC trace of modified peptide
with serine-octanoyl and aminohexanoic acid spacer.
Cyclic peptides
Cyclic peptides have drawn considerable interest because of their improved chemical stability and significantly enhanced resistance to in situ enzymatic clearance. The conformation is more rigid then the open-chain analog and may explain in part some of the observed improved biological activities. We routinely prepare cyclic peptides with head to tail ring closure. Directed cys-cys disulfide cyclization is also frequently achieved, and careful monitoring of the reaction ensures 100% cyclization.
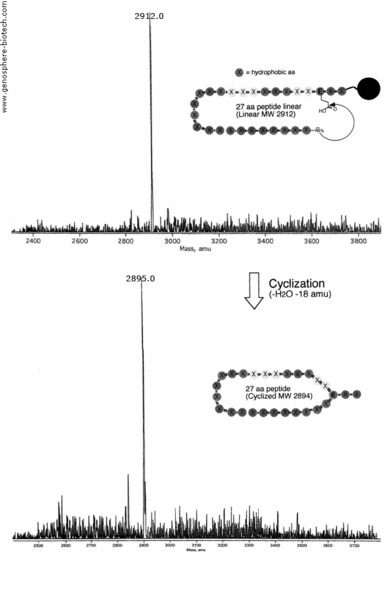
N-terminal amine to internal glutamic acid cyclization of a 27 aa peptide containing 21 hydrophobic residue (theoretical molar mass 2894) was prepared (100 mg) with purity level >95% and a sample analyzed by MALDI-TOF MS. MS shows loss of H2O (-18 amu) by condensation to yield desired product with molecular ion at m/z 2895.0.
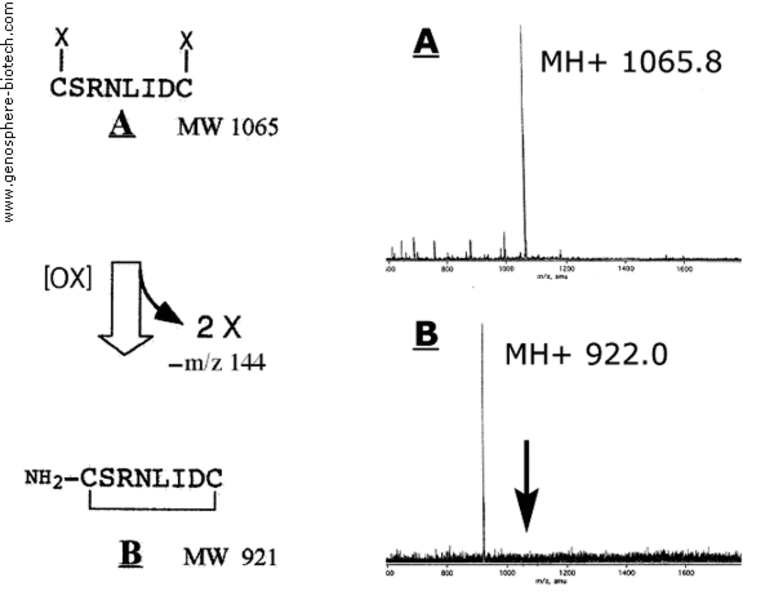
Mass spectra of A linear protected precursor
and B final cyclized peptide.
Labels and spacers
Depending upon your application we may prepare biotinylated or fluorescent peptides with various spacers. Aliphatic C3, C6, C12, C18 or hydrophilic polyethylene glycol (PEG)-derived spacers are available.
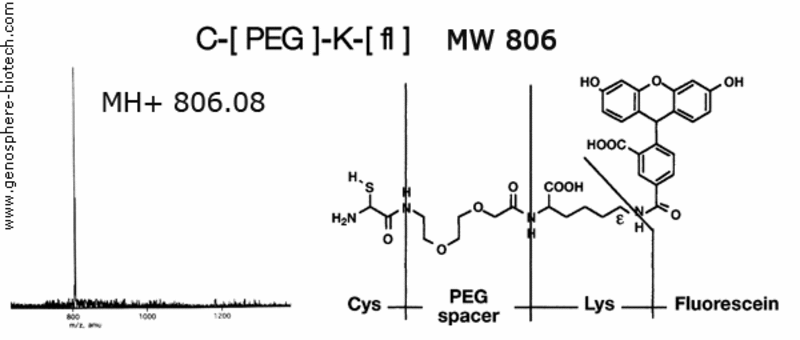
Mass spectrum of fluorescent peptide containing a PEG spacer.
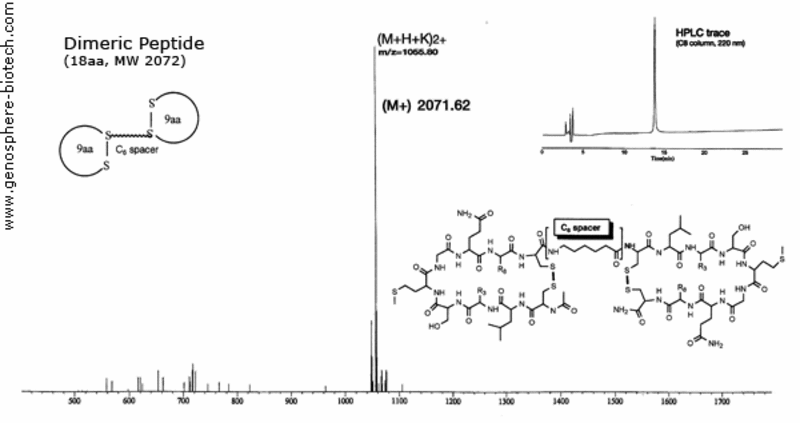
Mass spectrum and HPLC trace of a dimeric cyclic peptide linked with aminohexanoic acid spacer.
Peptide ends
Our peptides are normally synthesized with unblocked termini i.e. amine and carboxylic acid groups.
However in some instances, one might consider using blocking moieties, or modified ends.
N-acetyl and C-amide closely mimic internal sequences as they have uncharged ends. In addition, these blocking groups improve resistance to enzymatic degradation. It should be noted that solubility is decreased.
| N-terminal (default NH2) | C-terminal (default COOH) |
| acetyl, formyl | amide |
| myristoyl, palmitoyl | hydroxyl |
| carboxyl | thioester |
| 2-furoyl | (…) |
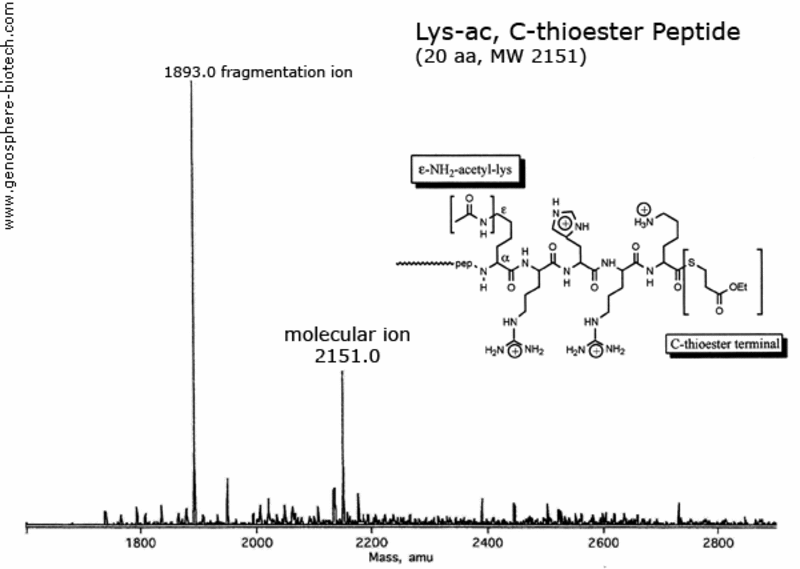
Mass spectrum of a 20 aa peptide with an acetylated lysine and thioester blocked C-end.
Many modifications available
Genosphere Biotechnologies provides a wide range of established peptide modification as exemplified below.
Many more are available, please don’t hesitate to contact us.
| Unusual Amino acids | Chemical Modifications |
| D-form | Phosphorylation: Tyr, Ser, Thr |
| Hydroxyproline | Biotin, DNP |
| Pyroglutamine | Anilide C-terminal |
| Methyl-, acetyl-lysine | Benzyloxycarbonylation |
| 4-Bromo-, 4-Nitro-phe | Nitrosation |
| beta-alanine | N-Methylation |
| Fluorescent labeling | Macromolecular Conjugation |
| Coumarin | Fatty acids |
| Fluorescein | Proteins: KLH, BSA, OVA |
| Rhodamine | Cyclisation |
| DANSYL | cys-cys disulfide bridge |
| NBD | Head to tail (N to C) cycle |
| DABCYL | Internal lactam, lactone |
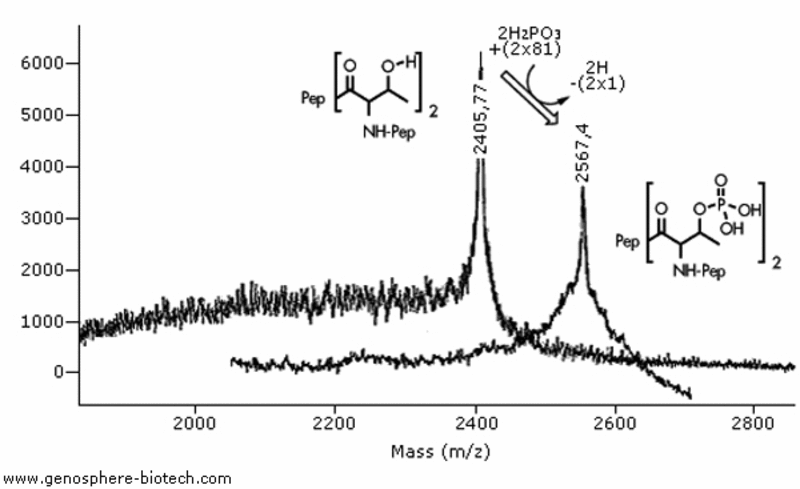
MALDI-TOF MS analysis of two 20-aa peptides with two threonine residues: (a) Unmodified peptide, theoretical molar mass of 2404 and (b) Peptide with phosphorylated threonines; theoretical molar mass 2564.
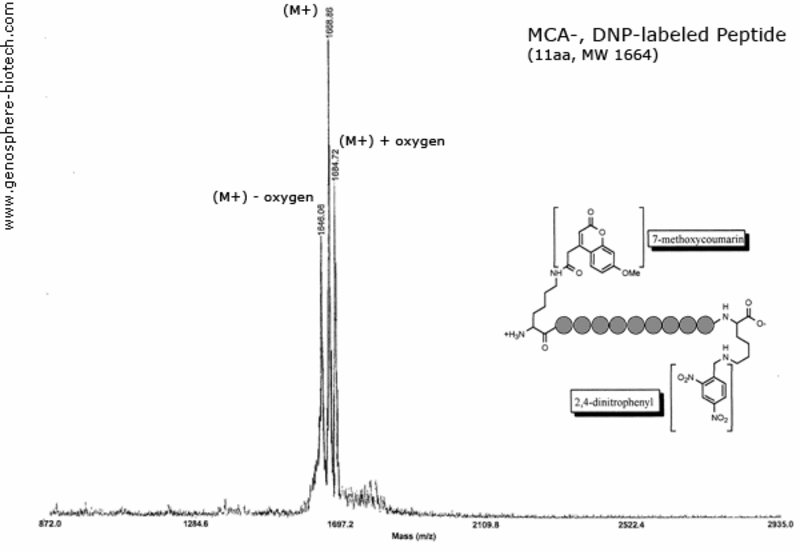
Mass spectrum of dual labeled peptide: 7-methoxycoumarin (MCA) and 2,4-dinitrophenyl (DNP) linked through the ε-amine of lysine residues.

