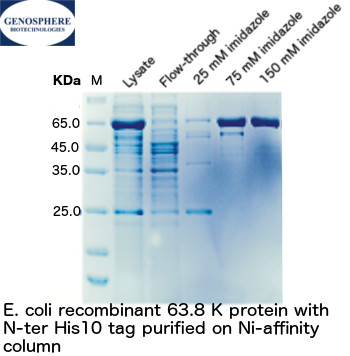
Protein expression in E. coli is the most economical and commonly used approach. It is particularly suitable for the preparation of antigens, ligands, cytokines or bacterial proteins. The ease of use of the system and the knowledge of host genetics have made it a system of choice in many instances. In addition, E. coli has a short culture period and offers the possibility of high yields.
Over the last decades, the E. coli expression system has been developed and documented by its extensive use in research and by industrial users for a large number of recombinant protein expressions.
Service description
All services include multiple steps from gene to purified protein as described in the table below.
Leadtime is approximately 8-10 weeks.
| Description | Resulting Material | Average turnaround (*) |
| Gene synthesis | ||
| - Codon optimisation - Gene synthesis - Cloning in standard pUC plasmid - Complete sequencing |
Cloned, synthesized and verified gene | 2 weeks |
| Subcloning for expression | ||
| - Plasmid prep - Subcloning - Clone selection - Complete sequencing |
Plasmid vector with cloned target gene | 2 weeks |
| Pilot expression | ||
| - Expression pilot and optimisation experiments - Choice of most suitable host - Adjust culture conditions - Determine yields and protein behavior |
10-50 µg of semi-purified protein | 2-3 weeks |
| Expression and purification | ||
| - Expression - Protein extraction - Protein purification - SDS-PAGE, WESTERN blot |
Purified protein | 2-3 weeks |
(*) Average production time for average difficult project. Actual turn around time varies with individual project features.