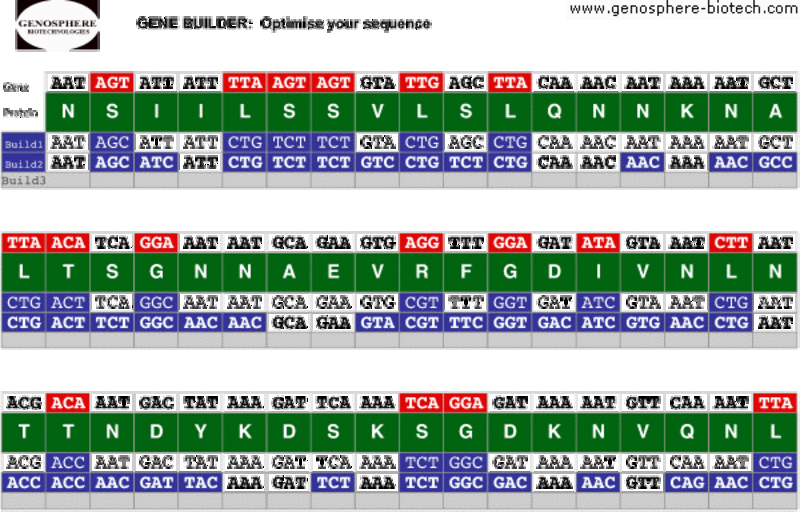
Pour éviter les conséquences dues à la répartition biaisée des codons, la séquence ADN originelle peut être redéfinie par changement de certaines séquences en utilisant la table de fréquence d’emploi des codons de l’organisme hôte : c’est l’optimisation des codons.
Il a été prouvé que cette procédure améliore considérablement l’expression hétérologue des protéines dans de nombreux cas. De plus, l’ingénierie des gènes par l’optimisation des codons permet de supprimer, le cas échéant, des éléments indésirables de la structure primaire tels que les séquences répétées ou les motifs pouvant entrainer la formation de structures secondaires ARNm stabilisées.
On peut également, si vous le souhaitez, ajouter des étiquettes pour la purification ou des sites de restriction ou encore plusieurs codons STOP.