Mass Spectrum
Mass spectrometry is defined (UIPAC) as the study of matter through the formation of gas-phase ions that are detected and characterized by their mass and charge.
In other words, it is an analytical technique that consists in weighing ions in the gas phase with the aim of establishing with high precision the masses of individual molecules.
Depending on the sample’s thermostability different ionization techniques are available:
Electron Impact (EI)
Chemical Ionization (CI)
Matrix Assisted Lazer Desorption Ionization (MALDI)
Electrospray Ionization (ESI)
Atmospheric Pressure Chemical Ionization (APCI)
Peptides are thermolabile molecules and require soft ionization techniques. The most popular approach is using ionisations techniques such as Electrospray (ESI) and Matrix Assisted Laser Desorption (MALDI). For MALDI, the MH+ species is usually the most dominant ion observed in the mass spectrum whereas ESI-MS yields multiple charged ions, [M+nH]n+.
Matrix Assisted Lazer Desorption Ionization (MALDI)
Some phenomenon should be kept in mind when using and interpreting MALDI-TOF MS. The extend of ionization and ejection in the gas phase of a given peptide will determine peak intensity. The latter is sequence-, mass- and sample-dependent. Some higher MW peptides may give small intensity Molecular Ion peak with this approach. An alternative approach is a higher-energy ionization techniques such as Electrospray Ionization. Another inherent phenomenon is the slight broadening of MH+ peaks resulting from very small local differences of absorbed energy during the initial UV-light laser pulses between similar peptide ions which in turn results in slight differences in Times of Flight and hence a broad spectrum. Routine calibration procedures afford average mass error of 0.1%.
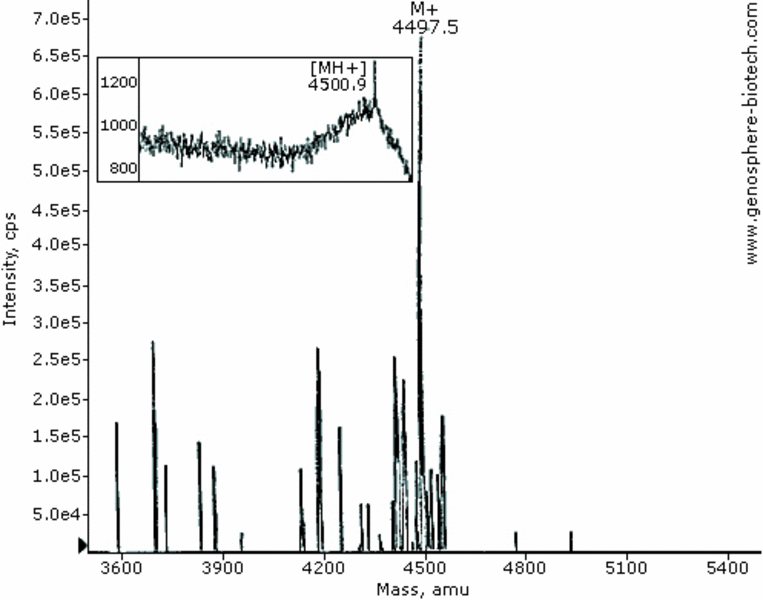
MALDI-TOF & ES MS of a 42-aa peptide (theoretical molar mass 4497).
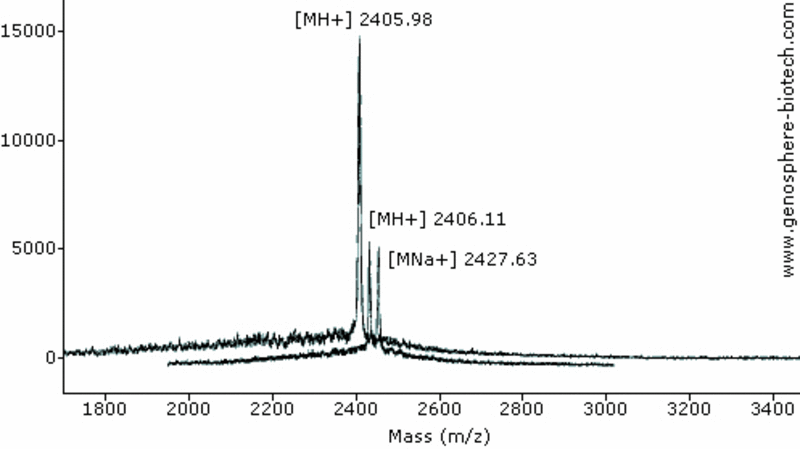
Two independent MALDI-TOF MS analysis. (a) 21-aa peptide (theoretical molar mass 2404).
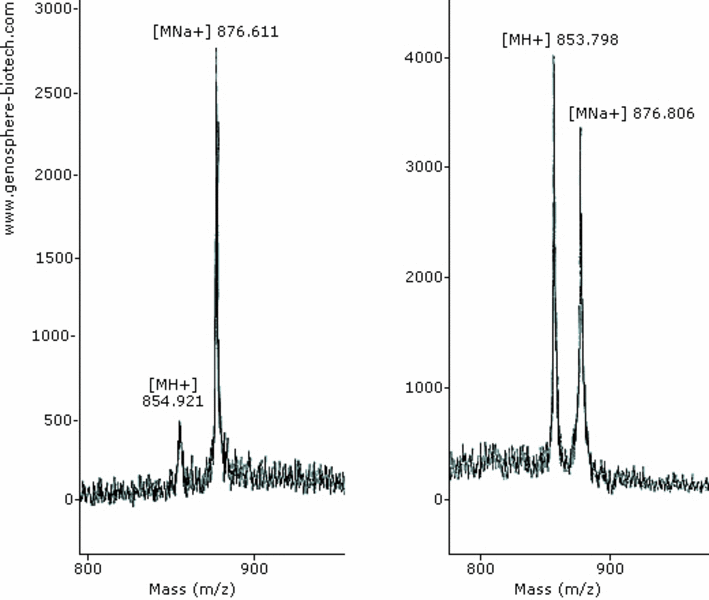
8-aa peptide (theoretical molar mass 854).
Electrospray Ionization (ESI)
In ESI-MS, analytes are introduced into the mass spectrometer in solution. The production of gas phase ions occurs under atmospheric pressure by producing charged droplets at a charged ES capillary tip. The shrinkage of the charged droplets due to solvent evaporation and repeated fission results in highly charged gas phase ions (protonation of the basic amino acids (Lys, Arg, His) and of the N-terminus) which can be separated according to their mass-to-charge ratio (m/z). To achieve protonation, the analytes must be basic both in solution (low pH) and in the gas phase. It is often expected that the primary sequence of the analytes and the pKb of the constituent ionisable amino groups of the peptide will be prominent factors in determining the number of protonation during positive ESI-MS analysis.
Although ESI mechanisms are still debated it is well-established that the observed ESI response (selectivity, charge state distribution (CSD) and signal intensity of ions in the resulting mass spectrum), is affected by instrumental parameters such as sheath gas flow rate or ES source geometry, by analyte concentration, solvent and analyte basicity, solvent surface tension, gas phase basicity and pH. Further, properties such as number and type of amino acids and conformation also play a role in determining the maximum obtainable charge state and CSD. For peptides, which have little secondary and tertiary structure, CSDs are independent of peptide secondary structure.
•Ehrmann, B. M.; Henriksen, T.; Cech, N. B., Relative importance of basicity in the gas phase and in solution for determining selectivity in electrospray ionization mass spectrometry. Journal of the American Society for Mass Spectrometry 2008, 19, 719-728
•Mansoori, B. A.; Volmer, D. A.; Boyd, R. K., ‘Wrong-way-round’ electrospray ionization of amino acids. Rapid Communications in Mass Spectrometry 1997, 11, 1120-1130
•Girod, M.; Dagany, X.; Antoine, R.; Dugourd, P., Relation between charge state distributions of peptide anions and pH changes in the electrospray plume. A mass spectrometry and optical spectroscopy investigation. International Journal of Mass Spectrometry 2011, 308, 41-48
•Winger, B. E.; Lightwahl, K. J.; Smith, R. D., Gas-phase proton-transfer reactions involving multiply charged cytochrome-c ions and water under thermal conditions. Journal of the American Society for Mass Spectrometry 1992, 3, 624-630
•Abaye, D. A.; Pullen, F. S.; Nielsen, B. V., Peptide polarity and the position of arginine as sources of selectivity during positive electrospray ionisation mass spectrometry. Rapid Communications in Mass Spectrometry 2011a, 25, 3597-3608
•Abaye, D. A.; Pullen, F. S.; Nielsen, B. V., Practical considerations in analysing neuropeptides, calcitonin gene-related peptide and vasoactive intestinal peptide, by nano-electrospray ionisation and quadrupole time-of-flight mass spectrometry: monitoring multiple protonations. Rapid Communications in Mass Spectrometry 2011b, 25, 1107-1116
•Tang, L.; Kebarle, P., Dependence of ion intensity in electrospray mass-spectrometry on the concentration of the analytes in the electrosprayed solution. Analytical Chemistry 1993, 65, 3654-3668
•Iavarone, A. T.; Jurchen, J. C.; Williams, E. R., Supercharged protein and peptide lone formed by electrospray ionization. Analytical Chemistry 2001, 73, 1455-1460
•Iavarone, A. T.; Williams, E. R., Mechanism of charging and supercharging molecules in electrospray ionization. Journal of the American Chemical Society 2003, 125, 2319-2327
•Carr, S. R.; Cassady, C. J., Reactivity and gas-phase acidity determinations of small peptide ions consisting of 11 to 14 amino acid residues. Journal of Mass Spectrometry 1997, 32, 959-967

